Key Insights
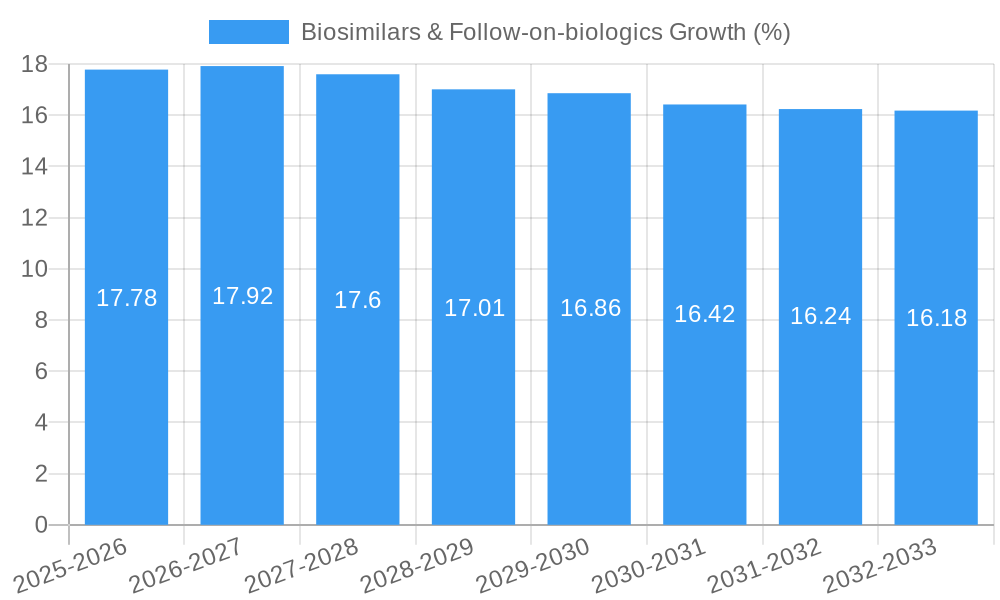
The global biosimilars and follow-on biologics market is experiencing robust expansion, poised for significant growth fueled by increasing healthcare demands and the strategic expiry of patents for blockbuster biologic drugs. While a precise market size for 2025 was not provided, industry projections suggest a valuation in the tens of billions of dollars, with an estimated Compound Annual Growth Rate (CAGR) of approximately 18-22% anticipated throughout the forecast period of 2025-2033. This rapid ascent is primarily driven by the imperative to reduce the financial burden of expensive biologic therapies, making them more accessible to a wider patient population. Key therapeutic areas contributing to this demand include oncology diseases, chronic and autoimmune diseases, and blood disorders, where biologics have revolutionized treatment paradigms. The increasing prevalence of these conditions globally further amplifies the need for cost-effective alternatives.
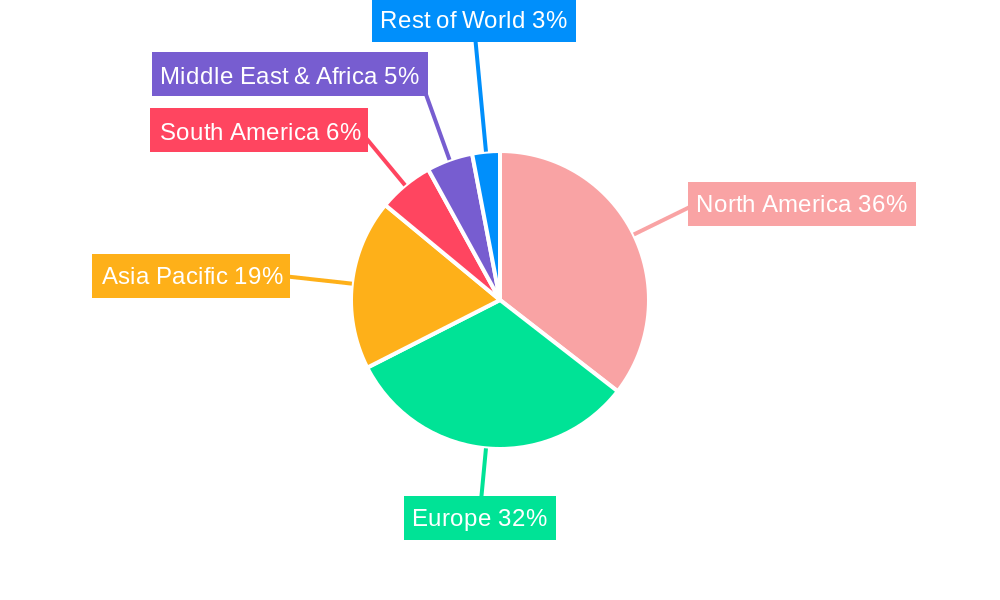
The market's trajectory is also shaped by evolving regulatory frameworks that facilitate the approval and uptake of biosimilars, alongside advancements in biopharmaceutical manufacturing technologies that enhance product quality and reduce production costs. The "Others" category within both application and type segments is likely to encompass emerging therapeutic areas and novel biologic drug classes, indicating the dynamic nature of this market. Key players like Novartis (Sandoz), Celltrion, and Teva Pharmaceuticals are actively investing in R&D and expanding their portfolios to capture a substantial share. North America and Europe currently dominate the market due to well-established healthcare systems and early adoption of biosimilars, but the Asia Pacific region is projected to witness the highest growth rates, driven by increasing healthcare expenditure, government initiatives to promote biosimilar use, and a large, underserved patient population. Restraints, such as complex regulatory pathways in certain regions and the lingering perception of biosimilars being inferior to originators, are being systematically addressed by manufacturers and regulatory bodies.
Biosimilars & Follow-on-Biologics Market Analysis Report: 2019-2033
This comprehensive report offers an in-depth analysis of the global biosimilars and follow-on-biologics market, providing critical insights for stakeholders navigating this rapidly evolving landscape. Covering the historical period of 2019-2024, base year of 2025, and an extensive forecast period from 2025 to 2033, this research delves into market dynamics, competitive strategies, and emerging trends. With a focus on high-impact applications such as oncology diseases, chronic and autoimmune diseases, and blood disorders, alongside key biologic types including monoclonal antibodies, erythropoietin, and human growth hormone, this report equips industry professionals with the actionable intelligence needed to capitalize on future opportunities.
Biosimilars & Follow-on-Biologics Market Structure & Competitive Dynamics
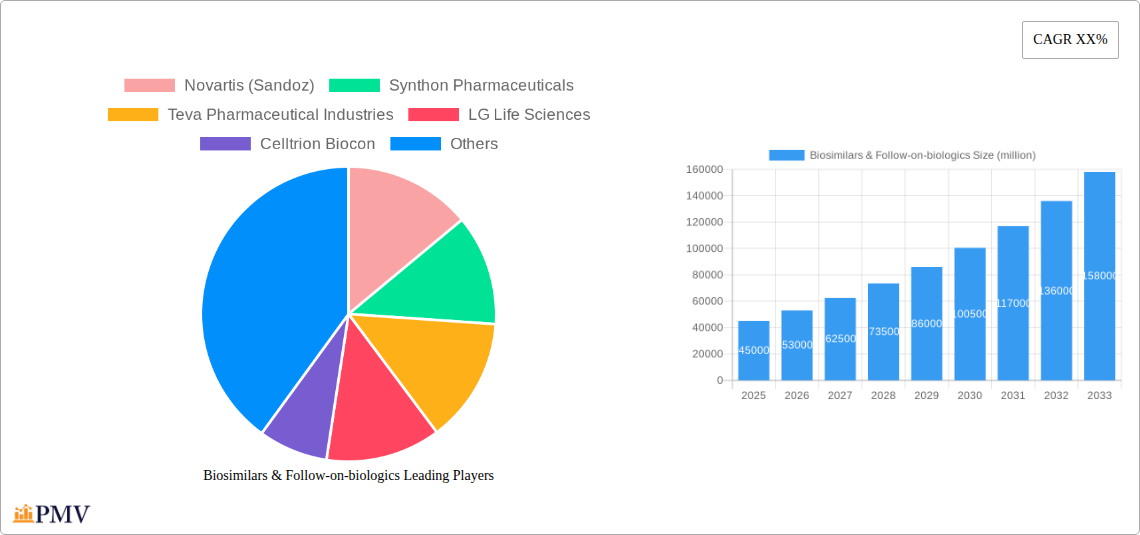
The global biosimilars and follow-on-biologics market exhibits a dynamic and increasingly competitive structure. Market concentration is moderate, with leading players investing heavily in R&D to secure intellectual property and expand their product pipelines. Innovation ecosystems are driven by collaborations between established pharmaceutical giants and specialized biosimilar developers, fostering rapid product development and regulatory approvals. Key players like Novartis (Sandoz), Synthon Pharmaceuticals, Teva Pharmaceutical Industries, LG Life Sciences, Celltrion Biocon, Hospira, Merck Serono (Merck), Biogen idec, and Genentech (Roche) are at the forefront, leveraging strategic partnerships and acquisitions. For instance, recent M&A activities have seen significant deal values, with reported figures reaching hundreds of millions. Regulatory frameworks, particularly in the US and Europe, play a crucial role in shaping market access and competition, with stringent approval pathways ensuring product efficacy and safety. The prevalence of product substitutes remains a significant factor, as originator biologics continue to hold a substantial market share, driven by brand loyalty and established clinical data. End-user trends are increasingly focused on cost-effectiveness without compromising therapeutic outcomes, directly fueling the demand for biosimilars.
Biosimilars & Follow-on-Biologics Industry Trends & Insights
The biosimilars and follow-on-biologics industry is experiencing robust growth, projected to witness a Compound Annual Growth Rate (CAGR) of approximately 18% from 2025 to 2033. This expansion is primarily driven by the expiry of patents for numerous blockbuster biologic drugs, creating significant opportunities for biosimilar developers. Technological advancements in biomanufacturing, including continuous processing and advanced analytical techniques, are reducing production costs and improving the quality of biosimilars, thereby enhancing their market penetration. The increasing global healthcare expenditure, coupled with rising prevalence of chronic diseases such as oncology diseases and chronic and autoimmune diseases, further fuels the demand for affordable biologic treatments. Consumer preferences are shifting towards biosimilars as healthcare providers and payers gain confidence in their therapeutic equivalence and cost-saving benefits. Competitive dynamics are intensifying, with established pharmaceutical companies increasingly focusing on biosimilar development to diversify their portfolios and offset declining revenues from off-patent small molecules. Furthermore, evolving regulatory pathways and market access policies in various regions are streamlining the approval and adoption of biosimilars, contributing to their broader market acceptance. The expanding therapeutic applications, particularly in areas like blood disorders and growth hormone deficiencies, are opening new avenues for market growth. Market penetration of biosimilars is expected to reach over 40% in developed markets by 2030, driven by significant cost savings for healthcare systems and patients alike.
Dominant Markets & Segments in Biosimilars & Follow-on-biologics
The North American region, particularly the United States, is anticipated to be a dominant market for biosimilars and follow-on-biologics due to its large healthcare expenditure, established regulatory framework, and high prevalence of target diseases. Within this region, oncology diseases are a key driver, representing a significant portion of biologic drug usage and consequently, a substantial market for biosimilars. The application segment of chronic and autoimmune diseases also exhibits strong growth potential, driven by increasing diagnosis rates and the availability of numerous biologic treatments for conditions like rheumatoid arthritis and inflammatory bowel disease.
Key drivers for dominance in this segment include:
- Favorable Economic Policies: Government initiatives aimed at reducing healthcare costs and promoting competition through biosimilar adoption.
- Robust Regulatory Pathways: Clear and efficient approval processes that facilitate the market entry of biosimilar products.
- High Healthcare Infrastructure: Advanced healthcare systems capable of adopting and administering complex biologic therapies.
- Significant Patient Population: A large patient base diagnosed with chronic and debilitating conditions requiring biologic intervention.
Among the types of biologics, monoclonal antibodies are expected to dominate the biosimilars market. This is attributed to their widespread use in treating a broad spectrum of diseases, including oncology diseases, chronic and autoimmune diseases, and inflammatory conditions. The high cost of originator monoclonal antibodies has created a significant unmet need for more affordable alternatives, making biosimilars a crucial solution. Erythropoietin and human growth hormone also represent substantial segments, particularly in treating blood disorders and growth hormone deficiencies, respectively. The market penetration in these segments is driven by established clinical evidence and increasing awareness among healthcare professionals and patients.
Biosimilars & Follow-on-Biologics Product Innovations
Product innovation in the biosimilars and follow-on-biologics sector is characterized by a focus on developing biosimilars for highly complex and high-value originator biologics. Companies are investing in advanced analytical techniques and sophisticated biomanufacturing processes to ensure product comparability and demonstrate efficacy. Key innovations involve the development of biosimilars for complex monoclonal antibodies used in oncology and immunology, offering significant cost savings without compromising therapeutic outcomes. This also includes the expansion of indications for existing biosimilars and the development of novel formulations, such as long-acting versions, to enhance patient convenience and adherence. The competitive advantage lies in achieving regulatory approval efficiently and establishing strong market access strategies.
Report Segmentation & Scope
This report meticulously segments the biosimilars and follow-on-biologics market by Application and Type.
Application Segments:
- Blood Disorders: This segment covers biosimilars for conditions like anemia, with significant market size and projected growth driven by established biosimilars of erythropoietin.
- Oncology Diseases: A major growth segment, driven by the high prevalence of cancer and the significant cost of originator biologic therapies.
- Chronic and Autoimmune Diseases: This segment includes conditions like rheumatoid arthritis and Crohn's disease, with substantial market potential due to the widespread use of monoclonal antibodies.
- Growth Hormone Deficiencies: A well-established segment with consistent demand for human growth hormone biosimilars.
- Others: Encompassing a diverse range of therapeutic areas.
Type Segments:
- Human Growth Hormone: A mature segment with steady demand.
- Erythropoietin: Crucial for treating anemia in various chronic conditions.
- Monoclonal Antibodies: The largest and fastest-growing segment, dominating therapeutic applications.
- Insulin: Essential for diabetes management, with growing biosimilar availability.
- Interferon: Used in treating viral infections and cancers.
- Granulocyte-Colony Stimulating Factor: Critical for managing neutropenia.
- Others: Including various other protein-based therapeutics.
Key Drivers of Biosimilars & Follow-on-Biologics Growth
The significant growth in the biosimilars and follow-on-biologics market is propelled by several interconnected factors. Patent expiries of blockbuster biologics are the primary catalyst, opening the door for generic competition. Cost containment pressures within healthcare systems globally are driving demand for more affordable treatment options, making biosimilars an attractive alternative. Advances in biotechnology and biomanufacturing have reduced development and production costs, enhancing the economic viability of biosimilar development. Furthermore, the supportive regulatory frameworks implemented by major health authorities are facilitating faster approvals and market access. The increasing clinical experience and physician confidence in biosimilar efficacy and safety are also crucial growth accelerators.
Challenges in the Biosimilars & Follow-on-Biologics Sector
Despite robust growth, the biosimilars and follow-on-biologics sector faces considerable challenges. Stringent and complex regulatory pathways can lead to lengthy approval times and high development costs. Extensive intellectual property litigation from originator companies can create significant barriers to market entry. Physician and patient education regarding biosimilar efficacy and interchangeability remains an ongoing effort. Supply chain complexities and manufacturing hurdles can impact production scale-up and consistent quality. Market access and reimbursement hurdles in certain regions can limit patient uptake. Additionally, competition from established originator biologics with strong brand recognition and long-standing clinical track records poses a constant challenge.
Leading Players in the Biosimilars & Follow-on-Biologics Market
- Novartis (Sandoz)
- Synthon Pharmaceuticals
- Teva Pharmaceutical Industries
- LG Life Sciences
- Celltrion
- Biocon
- Hospira
- Merck Serono (Merck)
- Biogen idec
- Genentech (Roche)
Key Developments in Biosimilars & Follow-on-Biologics Sector
- 2019: Significant increase in regulatory approvals for biosimilars targeting major blockbuster biologics in both the US and EU markets.
- 2020: Launch of several biosimilars for blockbuster monoclonal antibodies, intensifying competition in oncology and autoimmune disease segments.
- 2021: Strategic partnerships and M&A activities aimed at expanding manufacturing capabilities and R&D pipelines, with deal values exceeding hundreds of millions.
- 2022: Increased focus on developing biosimilars for more complex biologic molecules, requiring advanced analytical and manufacturing expertise.
- 2023: Expansion of biosimilar uptake in emerging markets, driven by increasing healthcare access and cost-consciousness.
- 2024: Introduction of novel biosimilars with improved pharmacokinetic profiles and extended half-lives, enhancing patient convenience.
Strategic Biosimilars & Follow-on-Biologics Market Outlook
The strategic outlook for the biosimilars and follow-on-biologics market remains exceptionally promising, fueled by a sustained pipeline of patent expiries and a growing global demand for affordable biologic therapies. Future growth will be accelerated by continued advancements in biomanufacturing technologies, leading to further cost reductions and improved product quality. Increased collaboration between biosimilar developers and healthcare providers will be crucial for enhancing market penetration and patient access. Strategic opportunities lie in targeting high-unmet-need therapeutic areas, developing complex biosimilars, and navigating evolving regulatory landscapes effectively. The market is poised for sustained expansion, with an estimated market size projected to reach over $100 billion by 2033, driven by a commitment to increasing patient access to life-saving biologic treatments.
Biosimilars & Follow-on-biologics Segmentation
-
1. Application
- 1.1. /> Blood Disorders
- 1.2. Oncology Diseases
- 1.3. Chronic and Autoimmune Diseases
- 1.4. Growth Hormone Deficiencies
- 1.5. Others
-
2. Type
- 2.1. /> Human growth hormone
- 2.2. Erythropoietin
- 2.3. Monoclonal antibodies
- 2.4. Insulin
- 2.5. Interferon
- 2.6. Granulocyte-Colony Stimulating Factor
- 2.7. Others
Biosimilars & Follow-on-biologics Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Biosimilars & Follow-on-biologics REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. /> Blood Disorders
- 5.1.2. Oncology Diseases
- 5.1.3. Chronic and Autoimmune Diseases
- 5.1.4. Growth Hormone Deficiencies
- 5.1.5. Others
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. /> Human growth hormone
- 5.2.2. Erythropoietin
- 5.2.3. Monoclonal antibodies
- 5.2.4. Insulin
- 5.2.5. Interferon
- 5.2.6. Granulocyte-Colony Stimulating Factor
- 5.2.7. Others
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. /> Blood Disorders
- 6.1.2. Oncology Diseases
- 6.1.3. Chronic and Autoimmune Diseases
- 6.1.4. Growth Hormone Deficiencies
- 6.1.5. Others
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. /> Human growth hormone
- 6.2.2. Erythropoietin
- 6.2.3. Monoclonal antibodies
- 6.2.4. Insulin
- 6.2.5. Interferon
- 6.2.6. Granulocyte-Colony Stimulating Factor
- 6.2.7. Others
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. /> Blood Disorders
- 7.1.2. Oncology Diseases
- 7.1.3. Chronic and Autoimmune Diseases
- 7.1.4. Growth Hormone Deficiencies
- 7.1.5. Others
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. /> Human growth hormone
- 7.2.2. Erythropoietin
- 7.2.3. Monoclonal antibodies
- 7.2.4. Insulin
- 7.2.5. Interferon
- 7.2.6. Granulocyte-Colony Stimulating Factor
- 7.2.7. Others
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. /> Blood Disorders
- 8.1.2. Oncology Diseases
- 8.1.3. Chronic and Autoimmune Diseases
- 8.1.4. Growth Hormone Deficiencies
- 8.1.5. Others
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. /> Human growth hormone
- 8.2.2. Erythropoietin
- 8.2.3. Monoclonal antibodies
- 8.2.4. Insulin
- 8.2.5. Interferon
- 8.2.6. Granulocyte-Colony Stimulating Factor
- 8.2.7. Others
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. /> Blood Disorders
- 9.1.2. Oncology Diseases
- 9.1.3. Chronic and Autoimmune Diseases
- 9.1.4. Growth Hormone Deficiencies
- 9.1.5. Others
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. /> Human growth hormone
- 9.2.2. Erythropoietin
- 9.2.3. Monoclonal antibodies
- 9.2.4. Insulin
- 9.2.5. Interferon
- 9.2.6. Granulocyte-Colony Stimulating Factor
- 9.2.7. Others
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Biosimilars & Follow-on-biologics Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. /> Blood Disorders
- 10.1.2. Oncology Diseases
- 10.1.3. Chronic and Autoimmune Diseases
- 10.1.4. Growth Hormone Deficiencies
- 10.1.5. Others
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. /> Human growth hormone
- 10.2.2. Erythropoietin
- 10.2.3. Monoclonal antibodies
- 10.2.4. Insulin
- 10.2.5. Interferon
- 10.2.6. Granulocyte-Colony Stimulating Factor
- 10.2.7. Others
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Novartis (Sandoz)
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Synthon Pharmaceuticals
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Teva Pharmaceutical Industries
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 LG Life Sciences
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Celltrion Biocon
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Hospira
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Merck Serono (Merck)
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Biogen idec
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Genentech (Roche)
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.1 Novartis (Sandoz)
List of Figures
- Figure 1: Global Biosimilars & Follow-on-biologics Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Biosimilars & Follow-on-biologics Revenue (million), by Application 2024 & 2032
- Figure 3: North America Biosimilars & Follow-on-biologics Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Biosimilars & Follow-on-biologics Revenue (million), by Type 2024 & 2032
- Figure 5: North America Biosimilars & Follow-on-biologics Revenue Share (%), by Type 2024 & 2032
- Figure 6: North America Biosimilars & Follow-on-biologics Revenue (million), by Country 2024 & 2032
- Figure 7: North America Biosimilars & Follow-on-biologics Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Biosimilars & Follow-on-biologics Revenue (million), by Application 2024 & 2032
- Figure 9: South America Biosimilars & Follow-on-biologics Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Biosimilars & Follow-on-biologics Revenue (million), by Type 2024 & 2032
- Figure 11: South America Biosimilars & Follow-on-biologics Revenue Share (%), by Type 2024 & 2032
- Figure 12: South America Biosimilars & Follow-on-biologics Revenue (million), by Country 2024 & 2032
- Figure 13: South America Biosimilars & Follow-on-biologics Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Biosimilars & Follow-on-biologics Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Biosimilars & Follow-on-biologics Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Biosimilars & Follow-on-biologics Revenue (million), by Type 2024 & 2032
- Figure 17: Europe Biosimilars & Follow-on-biologics Revenue Share (%), by Type 2024 & 2032
- Figure 18: Europe Biosimilars & Follow-on-biologics Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Biosimilars & Follow-on-biologics Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Biosimilars & Follow-on-biologics Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Biosimilars & Follow-on-biologics Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Biosimilars & Follow-on-biologics Revenue (million), by Type 2024 & 2032
- Figure 23: Middle East & Africa Biosimilars & Follow-on-biologics Revenue Share (%), by Type 2024 & 2032
- Figure 24: Middle East & Africa Biosimilars & Follow-on-biologics Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Biosimilars & Follow-on-biologics Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Biosimilars & Follow-on-biologics Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Biosimilars & Follow-on-biologics Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Biosimilars & Follow-on-biologics Revenue (million), by Type 2024 & 2032
- Figure 29: Asia Pacific Biosimilars & Follow-on-biologics Revenue Share (%), by Type 2024 & 2032
- Figure 30: Asia Pacific Biosimilars & Follow-on-biologics Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Biosimilars & Follow-on-biologics Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 4: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 7: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 13: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 19: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 31: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Type 2019 & 2032
- Table 40: Global Biosimilars & Follow-on-biologics Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Biosimilars & Follow-on-biologics Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Biosimilars & Follow-on-biologics?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Biosimilars & Follow-on-biologics?
Key companies in the market include Novartis (Sandoz), Synthon Pharmaceuticals, Teva Pharmaceutical Industries, LG Life Sciences, Celltrion Biocon, Hospira, Merck Serono (Merck), Biogen idec, Genentech (Roche).
3. What are the main segments of the Biosimilars & Follow-on-biologics?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4000.00, USD 6000.00, and USD 8000.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Biosimilars & Follow-on-biologics," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Biosimilars & Follow-on-biologics report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Biosimilars & Follow-on-biologics?
To stay informed about further developments, trends, and reports in the Biosimilars & Follow-on-biologics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



