Key Insights
The Japan Minimally Invasive Surgery (MIS) Devices market, valued at approximately ¥350 billion (assuming a conversion rate and market size approximation based on global trends and CAGR) in 2025, is projected to experience robust growth, driven by a rising elderly population, increasing prevalence of chronic diseases necessitating MIS procedures, and a strong focus on technological advancements within the Japanese healthcare system. The market's Compound Annual Growth Rate (CAGR) of 5.50% from 2025 to 2033 indicates a significant expansion, reaching an estimated ¥550 billion by 2033. Key growth drivers include the increasing adoption of robotic-assisted surgical systems, advanced imaging technologies enhancing surgical precision, and a growing preference for minimally invasive techniques among both surgeons and patients due to shorter recovery times and reduced scarring. Furthermore, government initiatives promoting technological advancements in healthcare and a well-established medical infrastructure in Japan contribute to this positive outlook. However, high device costs, stringent regulatory approvals, and the potential for reimbursement challenges could act as market restraints.
The market segmentation reveals significant opportunities across various product categories and applications. Robotic-assisted surgical systems, endoscopic devices, and laparoscopic devices are expected to witness substantial growth due to their superior capabilities and enhanced surgical outcomes. Cardiovascular, gastrointestinal, and orthopedic applications are leading segments, reflecting the high prevalence of related diseases in Japan's aging population. Regional variations exist, with Kanto, Kansai, and Chubu regions likely to dominate the market due to the concentration of major hospitals and medical research institutions. Leading players such as Smith & Nephew, Siemens Healthineers, Zimmer Biomet, and Medtronic PLC are actively engaged in expanding their presence in the Japanese market through strategic partnerships, product launches, and technological innovations. This competitive landscape fosters ongoing improvements in product offerings and further fuels market growth.
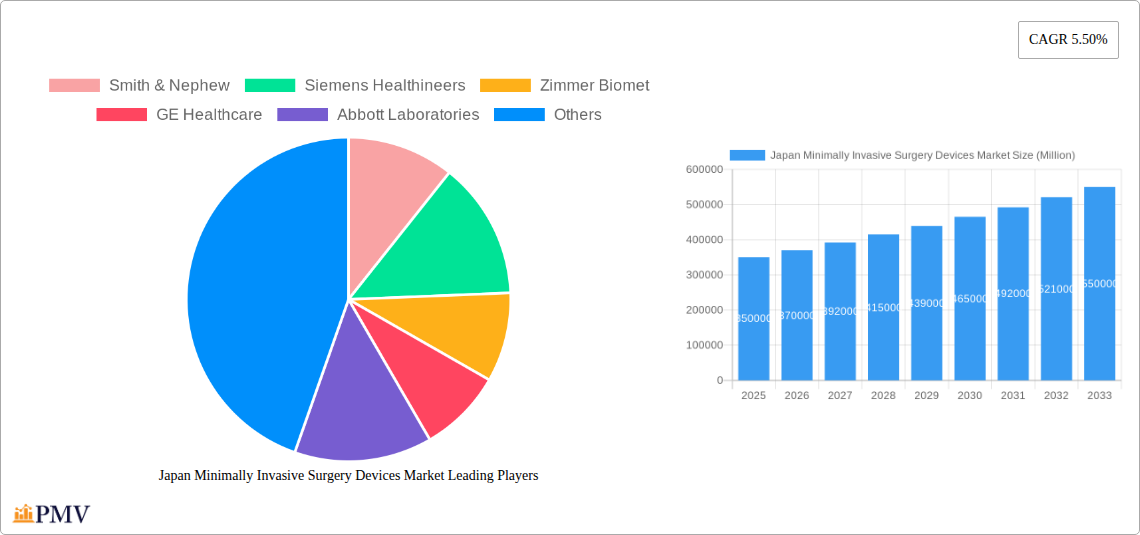
Japan Minimally Invasive Surgery (MIS) Devices Market Report: 2019-2033
This comprehensive report provides an in-depth analysis of the Japan Minimally Invasive Surgery Devices market, offering valuable insights for stakeholders including manufacturers, investors, and healthcare professionals. Covering the period 2019-2033, with a focus on 2025, this report unveils the market's structure, competitive landscape, growth drivers, challenges, and future outlook. The report segments the market by product (Handheld Instruments, Guiding Devices, Electrosurgical Devices, Endoscopic Devices, Laproscopic Devices, Monitoring and Visualization Devices, Robotic Assisted Surgical Systems, Ablation Devices, Laser Based Devices, Other MIS Devices) and application (Cardiovascular, Gastrointestinal, Gynecological, Orthopedic, Urological, Other Applications), providing granular data and forecasts.
Japan Minimally Invasive Surgery Devices Market Market Structure & Competitive Dynamics
The Japan MIS devices market exhibits a moderately concentrated structure, with key players like Smith & Nephew, Siemens Healthineers, Zimmer Biomet, GE Healthcare, Abbott Laboratories, Medtronic PLC, Koninklijke Philips NV, Intuitive Surgical Inc, Stryker Corporation, and Olympus Corporation holding significant market share. The market is characterized by a dynamic innovation ecosystem driven by advancements in robotics, imaging, and materials science. Stringent regulatory frameworks, including those set by the Pharmaceuticals and Medical Devices Agency (PMDA), influence product approvals and market entry. The presence of substitute therapies and evolving end-user preferences (e.g., minimally invasive procedures) further shape the competitive landscape. M&A activities, though not exceptionally frequent, play a role in consolidating market share and enhancing technological capabilities. Over the historical period (2019-2024), M&A deal values totalled approximately xx Million, with an average deal size of xx Million. Market share for the top 5 players combined was estimated at xx% in 2024.
Japan Minimally Invasive Surgery Devices Market Industry Trends & Insights
The Japan MIS devices market is projected to experience significant growth during the forecast period (2025-2033), driven by an aging population, rising prevalence of chronic diseases requiring minimally invasive treatment, and increasing government initiatives promoting advanced healthcare technologies. The market's Compound Annual Growth Rate (CAGR) is estimated at xx% from 2025 to 2033. Technological disruptions, such as the integration of artificial intelligence (AI) and augmented reality (AR) in surgical procedures, are accelerating market expansion. Consumer preferences are shifting towards less invasive procedures with faster recovery times and reduced hospital stays. Intense competition among established players and emerging companies is spurring innovation and product diversification. Market penetration of robotic-assisted surgical systems, although currently low, is expected to increase substantially due to their enhanced precision and effectiveness. The increasing adoption of single-site and natural orifice transluminal endoscopic surgery (NOTES) procedures further contributes to market growth, with expected market penetration reaching xx% by 2033.
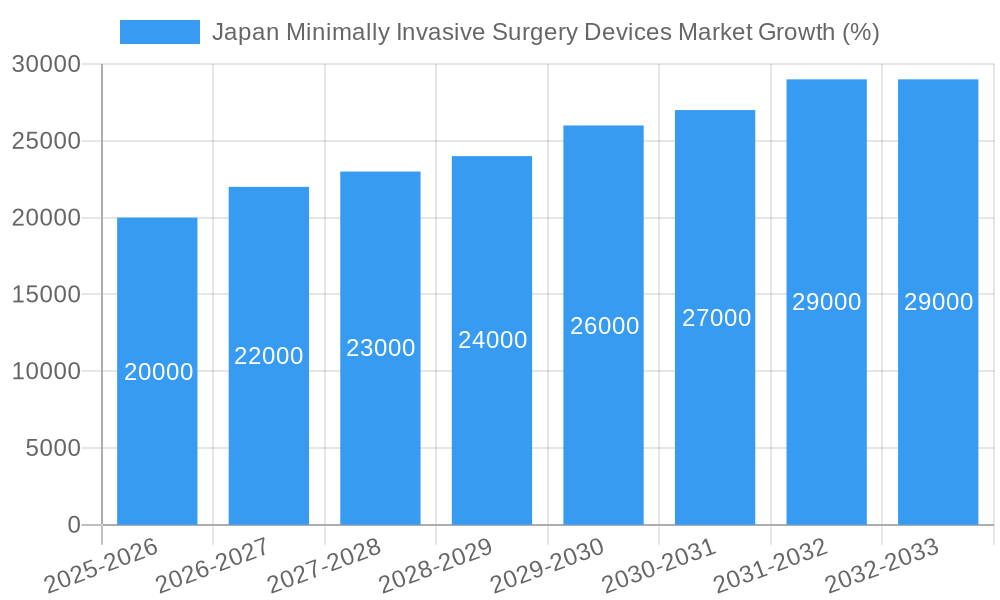
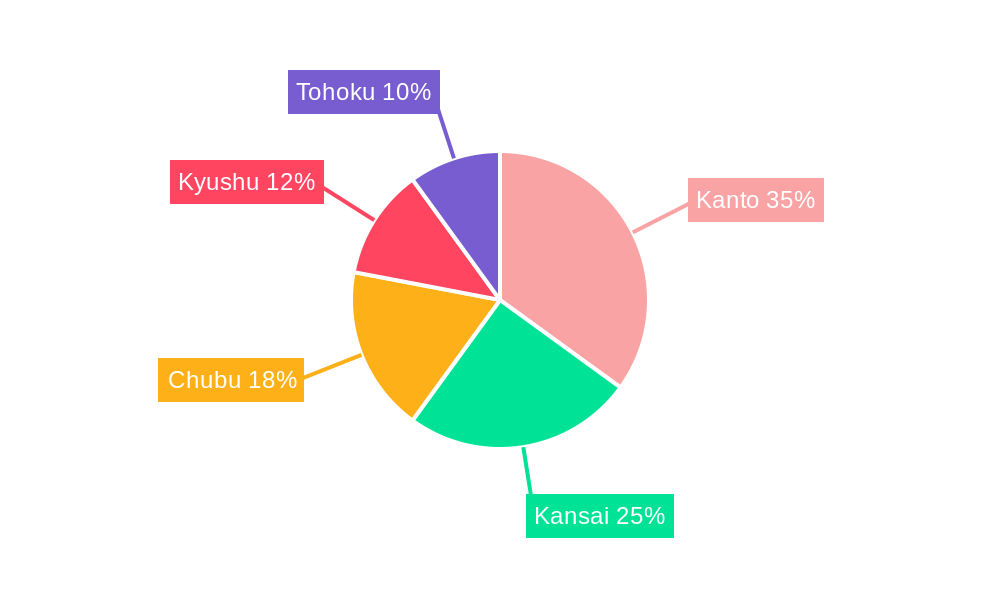
Dominant Markets & Segments in Japan Minimally Invasive Surgery Devices Market
Within the Japanese MIS devices market, the orthopedic segment currently holds the largest share by application, driven by the high incidence of orthopedic conditions among the aging population and increasing demand for minimally invasive joint replacement surgeries. The endoscopic devices segment dominates the product category due to the widespread use of endoscopic procedures across various surgical specialties.
- Key Drivers for Orthopedic Segment Dominance:
- High prevalence of osteoarthritis and other degenerative joint diseases.
- Increasing adoption of minimally invasive orthopedic techniques.
- Favorable reimbursement policies for MIS orthopedic procedures.
- Key Drivers for Endoscopic Devices Segment Dominance:
- Wide applicability across various surgical specialties.
- Technological advancements enhancing image quality and precision.
- Growing preference for minimally invasive procedures with reduced recovery time.
The Kanto region, encompassing Tokyo and surrounding prefectures, represents the largest regional market due to its high concentration of hospitals, medical institutions, and skilled surgical professionals. Government initiatives promoting medical tourism also contribute to the region's market dominance.
Japan Minimally Invasive Surgery Devices Market Product Innovations
Recent innovations in Japan's MIS device market include the development of smaller, more precise instruments, improved imaging technologies for enhanced visualization, and the integration of AI for surgical planning and assistance. These advancements enhance surgical precision, minimize invasiveness, and improve patient outcomes. The market is witnessing increased adoption of robotic-assisted surgical systems, particularly in complex procedures, highlighting the growing emphasis on technology-driven solutions. The focus is on designing devices for improved ergonomics, enhanced usability, and reduced complications.
Report Segmentation & Scope
This report segments the Japan MIS devices market comprehensively.
By Product: Handheld Instruments, Guiding Devices, Electrosurgical Devices, Endoscopic Devices, Laproscopic Devices, Monitoring and Visualization Devices, Robotic Assisted Surgical Systems, Ablation Devices, Laser Based Devices, Other MIS Devices. Each segment's growth projections, market size, and competitive dynamics are analyzed, revealing the robotic assisted surgical systems segment showing the highest growth projection.
By Application: Cardiovascular, Gastrointestinal, Gynecological, Orthopedic, Urological, Other Applications. The report provides detailed analysis of each application segment, revealing orthopedic surgeries leading in market size.
Key Drivers of Japan Minimally Invasive Surgery Devices Market Growth
Several factors propel the growth of Japan's MIS devices market. The aging population significantly increases the demand for surgical procedures. Government initiatives promoting technological advancements in healthcare and favorable reimbursement policies for MIS procedures further stimulate market growth. The rising prevalence of chronic diseases requiring less invasive treatments, coupled with increasing patient preference for minimally invasive procedures, contributes to the market expansion.
Challenges in the Japan Minimally Invasive Surgery Devices Market Sector
Despite the market's promising outlook, challenges persist. Stringent regulatory approvals can delay product launches, impacting market entry. High device costs can limit accessibility, particularly for patients with limited insurance coverage. Intense competition from both domestic and international players necessitates continuous innovation to maintain a competitive edge. The supply chain vulnerabilities also need to be managed effectively. These factors collectively represent hurdles that market participants must navigate.
Leading Players in the Japan Minimally Invasive Surgery Devices Market Market
- Smith & Nephew
- Siemens Healthineers
- Zimmer Biomet
- GE Healthcare
- Abbott Laboratories
- Medtronic PLC
- Koninklijke Philips NV
- Intuitive Surgical Inc
- Stryker Corporation
- Olympus Corporation
Key Developments in Japan Minimally Invasive Surgery Devices Market Sector
- 2023-Q4: Olympus Corporation launches a new generation of endoscopic devices with enhanced visualization capabilities.
- 2022-Q2: Medtronic PLC receives PMDA approval for a new robotic-assisted surgical system.
- 2021-Q1: A strategic partnership between Smith & Nephew and a Japanese medical technology company accelerates product development in the MIS area. (Further specific details of other developments would be included in the full report)
Strategic Japan Minimally Invasive Surgery Devices Market Market Outlook
The future of the Japan MIS devices market looks exceptionally promising. Continued technological advancements, particularly in robotics and AI, coupled with a supportive regulatory environment and growing demand for minimally invasive procedures, are expected to drive substantial market growth. Strategic partnerships and collaborations, focusing on innovation and product diversification, will be key to success in this competitive market. Companies focusing on cost-effective solutions and improved accessibility will secure a competitive advantage.
Japan Minimally Invasive Surgery Devices Market Segmentation
-
1. Product
- 1.1. Handheld Instruments
- 1.2. Guiding Devices
- 1.3. Electrosurgical Devices
- 1.4. Endoscopic Devices
- 1.5. Laproscopic Devices
- 1.6. Monitoring and Visualization Devices
- 1.7. Robotic Assisted Surgical Systems
- 1.8. Ablation Devices
- 1.9. Laser Based Devices
- 1.10. Other MIS Devices
-
2. Application
- 2.1. Cardiovascular
- 2.2. Gastrointestinal
- 2.3. Gynecological
- 2.4. Orthopedic
- 2.5. Urological
- 2.6. Other Applications
Japan Minimally Invasive Surgery Devices Market Segmentation By Geography
- 1. Japan
Japan Minimally Invasive Surgery Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Lifestyle-related and Chronic Disorders; Technological Advancements
- 3.3. Market Restrains
- 3.3.1. Shortage of Experienced Professionals
- 3.4. Market Trends
- 3.4.1. Gastrointestinal Segment Expects to Register a High CAGR Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Product
- 5.1.1. Handheld Instruments
- 5.1.2. Guiding Devices
- 5.1.3. Electrosurgical Devices
- 5.1.4. Endoscopic Devices
- 5.1.5. Laproscopic Devices
- 5.1.6. Monitoring and Visualization Devices
- 5.1.7. Robotic Assisted Surgical Systems
- 5.1.8. Ablation Devices
- 5.1.9. Laser Based Devices
- 5.1.10. Other MIS Devices
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Cardiovascular
- 5.2.2. Gastrointestinal
- 5.2.3. Gynecological
- 5.2.4. Orthopedic
- 5.2.5. Urological
- 5.2.6. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Japan
- 5.1. Market Analysis, Insights and Forecast - by Product
- 6. Kanto Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 7. Kansai Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 8. Chubu Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 9. Kyushu Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 10. Tohoku Japan Minimally Invasive Surgery Devices Market Analysis, Insights and Forecast, 2019-2031
- 11. Competitive Analysis
- 11.1. Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Smith & Nephew
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Siemens Healthineers
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Zimmer Biomet
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 GE Healthcare
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Abbott Laboratories
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Medtronic PLC
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Koninklijke Philips NV
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Intuitive Surgical Inc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Stryker Corporation
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Olympus Corporation
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Smith & Nephew
List of Figures
- Figure 1: Japan Minimally Invasive Surgery Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Japan Minimally Invasive Surgery Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Product 2019 & 2032
- Table 4: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Product 2019 & 2032
- Table 5: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 6: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 7: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 8: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 9: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 11: Kanto Japan Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Kanto Japan Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 13: Kansai Japan Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Kansai Japan Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 15: Chubu Japan Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Chubu Japan Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 17: Kyushu Japan Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Kyushu Japan Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 19: Tohoku Japan Minimally Invasive Surgery Devices Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Tohoku Japan Minimally Invasive Surgery Devices Market Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 21: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Product 2019 & 2032
- Table 22: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Product 2019 & 2032
- Table 23: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 24: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 25: Japan Minimally Invasive Surgery Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 26: Japan Minimally Invasive Surgery Devices Market Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Japan Minimally Invasive Surgery Devices Market?
The projected CAGR is approximately 5.50%.
2. Which companies are prominent players in the Japan Minimally Invasive Surgery Devices Market?
Key companies in the market include Smith & Nephew, Siemens Healthineers, Zimmer Biomet, GE Healthcare, Abbott Laboratories, Medtronic PLC, Koninklijke Philips NV, Intuitive Surgical Inc, Stryker Corporation, Olympus Corporation.
3. What are the main segments of the Japan Minimally Invasive Surgery Devices Market?
The market segments include Product, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Lifestyle-related and Chronic Disorders; Technological Advancements.
6. What are the notable trends driving market growth?
Gastrointestinal Segment Expects to Register a High CAGR Over the Forecast Period.
7. Are there any restraints impacting market growth?
Shortage of Experienced Professionals.
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Japan Minimally Invasive Surgery Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Japan Minimally Invasive Surgery Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Japan Minimally Invasive Surgery Devices Market?
To stay informed about further developments, trends, and reports in the Japan Minimally Invasive Surgery Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



