Key Insights
The Canadian artificial organs and bionics market is experiencing robust growth, driven by an aging population, increasing prevalence of chronic diseases necessitating organ replacement or augmentation, and advancements in medical technology. The 6.30% CAGR (2019-2033) indicates a significant expansion, with the market expected to reach a substantial size by 2033. This growth is fueled by several key factors. Firstly, technological innovations are leading to the development of more sophisticated and effective artificial organs and bionic devices, improving patient outcomes and extending lifespans. Secondly, rising healthcare expenditure and improved healthcare infrastructure in Canada are contributing to greater accessibility of these advanced medical solutions. Furthermore, government initiatives promoting medical innovation and supporting research in this sector are fostering market expansion. The market segmentation reveals significant potential within both artificial organ replacement (e.g., heart, kidney, etc.) and bionics (prosthetics, implants for sensory augmentation). While precise market values for specific years are not provided, a conservative estimation using the given CAGR suggests substantial growth from the 2025 base year.
The market faces certain challenges, though. High costs associated with artificial organs and bionic devices create a significant barrier to access for many patients. Regulatory hurdles and the rigorous approval processes for new technologies can slow down market penetration. Moreover, ethical considerations surrounding the use of artificial organs and bionics, and potential long-term complications, require careful management. The regional distribution of the market across Eastern, Central, and Western Canada likely reflects variations in population density, healthcare infrastructure, and economic factors. However, the presence of major players like Ossur, Medtronic, and Boston Scientific Corporation signals a strong competitive landscape and significant investment in the sector's future in Canada. The forecast period (2025-2033) promises continued expansion, driven by ongoing technological progress, improving affordability, and increased public awareness of the benefits of artificial organs and bionic solutions.
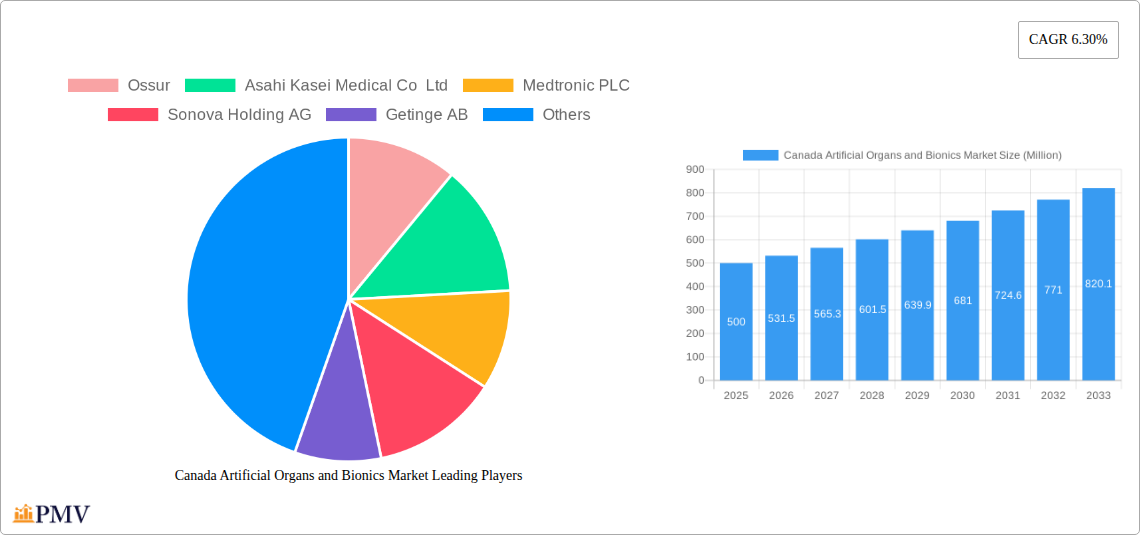
Canada Artificial Organs and Bionics Market: A Comprehensive Report (2019-2033)
This in-depth report provides a comprehensive analysis of the Canada Artificial Organs and Bionics Market, offering invaluable insights for stakeholders across the healthcare industry. Covering the period 2019-2033, with a base year of 2025 and a forecast period of 2025-2033, this report meticulously examines market structure, competitive dynamics, industry trends, and future growth prospects. The report segments the market by Type (Artificial Organ, Bionics), providing detailed analysis of market size, growth projections, and key players. The total market value is predicted to reach xx Million by 2033.
Canada Artificial Organs and Bionics Market Market Structure & Competitive Dynamics
The Canadian artificial organs and bionics market exhibits a moderately concentrated structure, with a handful of multinational corporations holding significant market share. Key players such as Ossur, Asahi Kasei Medical Co Ltd, Medtronic PLC, Sonova Holding AG, and Getinge AB dominate the landscape. However, the market also features several smaller, specialized companies focusing on niche areas within bionics, fostering a dynamic competitive environment.
Market share is largely determined by factors such as technological innovation, regulatory approvals, distribution networks, and brand reputation. The market concentration ratio (CR4) is estimated at xx%, indicating moderate concentration. Mergers and acquisitions (M&A) activity has been moderate in recent years, with deal values ranging from xx Million to xx Million. These transactions primarily involve smaller companies being acquired by larger players to expand their product portfolios or gain access to new technologies. Regulatory frameworks, including Health Canada's approval processes, significantly influence market entry and product availability. The market is also subject to influences from product substitutes (e.g., conservative treatment options) and evolving end-user preferences (e.g., increasing demand for minimally invasive procedures and improved patient outcomes).
Canada Artificial Organs and Bionics Market Industry Trends & Insights
The Canadian artificial organs and bionics market is experiencing robust growth, driven by several key factors. The aging population, increasing prevalence of chronic diseases requiring artificial organs or bionic devices (e.g., heart failure, hearing loss, limb loss), and rising disposable incomes are major contributors to market expansion. Technological advancements, such as the development of more biocompatible materials, miniaturization of devices, and improved surgical techniques, are further accelerating market growth.
The market is also witnessing significant technological disruptions, with the emergence of AI-powered diagnostic tools, personalized medicine approaches, and advanced bioprinting techniques transforming the sector. Consumer preferences are shifting towards minimally invasive procedures, improved cosmetic outcomes, and enhanced device functionality. This is leading to increased demand for innovative products with improved performance and longevity. The Compound Annual Growth Rate (CAGR) for the forecast period (2025-2033) is estimated to be xx%, with market penetration steadily increasing, particularly in urban areas. Competitive dynamics remain intense, with companies focusing on innovation, strategic partnerships, and targeted marketing campaigns to gain market share.
Dominant Markets & Segments in Canada Artificial Organs and Bionics Market
The Bionics segment currently holds a larger market share compared to the Artificial Organ segment in Canada. This dominance is attributed to:
- Higher Prevalence of Treatable Conditions: Hearing loss and other conditions treatable with bionic devices affect a larger population compared to those requiring artificial organs.
- Technological Advancements: Rapid advancements in areas like cochlear implants and neuromodulation have led to improved device efficacy and patient outcomes, stimulating demand.
- Increased Affordability: Technological advancements have also led to cost reductions in certain bionic devices, making them accessible to a larger patient population.
- Strong Regulatory Support: The regulatory landscape in Canada encourages the adoption of innovative bionic solutions, providing a favorable environment for market growth.
Key Drivers for Bionics segment dominance:
- Increased government funding for healthcare research and innovation.
- Growing awareness and acceptance of bionic devices among the population.
- Robust private healthcare insurance coverage for bionic procedures.
Canada Artificial Organs and Bionics Market Product Innovations
Recent product developments in the Canadian market emphasize miniaturization, improved biocompatibility, and enhanced functionality. For example, the launch of the Axonics F15 sacral neuromodulation system and the ReSound OMNIA hearing aids represent significant technological advancements that address unmet needs. These innovations enhance patient comfort, improve efficacy, and contribute to better long-term outcomes. The focus is on seamlessly integrating devices with the human body for improved quality of life.
Report Segmentation & Scope
The report segments the Canada Artificial Organs and Bionics Market by Type: Artificial Organ (e.g., artificial heart, kidney, liver) and Other Organ Types: Bionics (e.g., cochlear implants, prosthetic limbs, retinal implants). Each segment's growth projections, market size, and competitive dynamics are thoroughly analyzed. The Artificial Organ segment is expected to witness a CAGR of xx% during the forecast period, driven by increasing prevalence of organ failure and advancements in organ transplantation techniques. The Bionics segment is projected to achieve a CAGR of xx%, propelled by technological advancements and rising demand for improved quality of life solutions.
Key Drivers of Canada Artificial Organs and Bionics Market Growth
The Canadian artificial organs and bionics market's growth is primarily driven by technological advancements, favorable government policies promoting healthcare innovation, and a growing aging population necessitating these devices. The rising prevalence of chronic diseases such as heart failure, diabetes, and hearing loss fuels demand for artificial organs and bionics. Furthermore, substantial investment in research and development, leading to improved product efficacy and safety, is bolstering market expansion.
Challenges in the Canada Artificial Organs and Bionics Market Sector
Significant challenges exist in this market. High cost of devices, limited insurance coverage, and lengthy regulatory approval processes impede market growth. Supply chain disruptions, particularly for specialized components, can also restrict production and availability. The highly competitive landscape necessitates continuous innovation and strategic partnerships to maintain a strong market position. These factors collectively affect market expansion and access to life-changing technology.
Leading Players in the Canada Artificial Organs and Bionics Market Market
- Ossur
- Asahi Kasei Medical Co Ltd
- Medtronic PLC
- Sonova Holding AG
- Getinge AB
- Ekso Bionics Holdings Inc
- Boston Scientific Corporation
- Baxter International Inc
- Berlin Heart GmbH
- Med-El Elektromedizinische Gerate GmbH
Key Developments in Canada Artificial Organs and Bionics Market Sector
- September 2022: Axonics completes the first implant of its Axonics F15 sacral neuromodulation (SNM) system in Canada, marking a significant advancement in treating bladder and bowel dysfunction.
- August 2022: GN Hearing launches new ReSound OMNIA hearing aids and Beltone Achieve line-up in Canada, enhancing the hearing aid market with advanced Receiver-in-Ear technology.
Strategic Canada Artificial Organs and Bionics Market Market Outlook
The future of the Canadian artificial organs and bionics market appears promising. Continued technological advancements, coupled with growing government support and increasing awareness among patients, are expected to drive significant market expansion. Strategic opportunities exist for companies focusing on innovation, personalized medicine, and digital health integration. The market offers substantial potential for both established players and new entrants who can successfully navigate the regulatory landscape and address unmet clinical needs.
Canada Artificial Organs and Bionics Market Segmentation
-
1. Type
-
1.1. Artificial Organ
- 1.1.1. Artificial Heart
- 1.1.2. Artificial Kidneys
- 1.1.3. Artificial Lungs
- 1.1.4. Cochlear Implants
- 1.1.5. Other Organ Types
-
1.2. Bionics
- 1.2.1. Vision Bionics
- 1.2.2. Ear Bionics
- 1.2.3. Orthopedic Bionic
- 1.2.4. Cardiac Bionics
-
1.1. Artificial Organ
Canada Artificial Organs and Bionics Market Segmentation By Geography
- 1. Canada
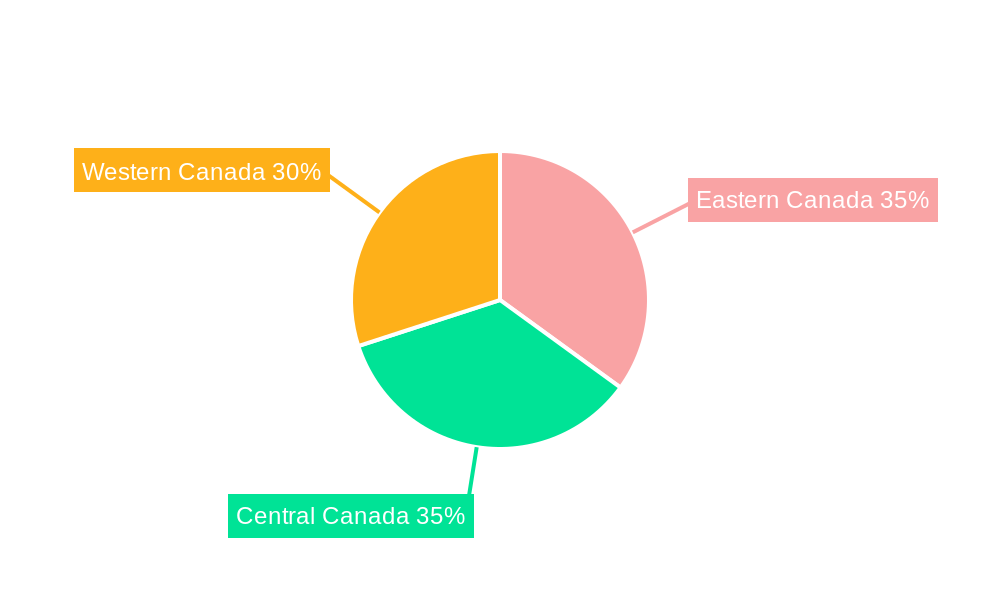
Canada Artificial Organs and Bionics Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.30% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Incidences of Organ Failure; Scarcity of Donor Organs
- 3.3. Market Restrains
- 3.3.1. High Cost of Procedure; Risk of Compatibility and Malfunctions
- 3.4. Market Trends
- 3.4.1. Artificial Kidney Segment is Expected to Witness Significant Growth During the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Canada Artificial Organs and Bionics Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Artificial Organ
- 5.1.1.1. Artificial Heart
- 5.1.1.2. Artificial Kidneys
- 5.1.1.3. Artificial Lungs
- 5.1.1.4. Cochlear Implants
- 5.1.1.5. Other Organ Types
- 5.1.2. Bionics
- 5.1.2.1. Vision Bionics
- 5.1.2.2. Ear Bionics
- 5.1.2.3. Orthopedic Bionic
- 5.1.2.4. Cardiac Bionics
- 5.1.1. Artificial Organ
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Canada
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Eastern Canada Canada Artificial Organs and Bionics Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 6.1.1. undefined
- 7. Central Canada Canada Artificial Organs and Bionics Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 7.1.1. undefined
- 8. Western Canada Canada Artificial Organs and Bionics Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 8.1.1. undefined
- 9. Competitive Analysis
- 9.1. Market Share Analysis 2024
- 9.2. Company Profiles
- 9.2.1 Ossur
- 9.2.1.1. Overview
- 9.2.1.2. Products
- 9.2.1.3. SWOT Analysis
- 9.2.1.4. Recent Developments
- 9.2.1.5. Financials (Based on Availability)
- 9.2.2 Asahi Kasei Medical Co Ltd
- 9.2.2.1. Overview
- 9.2.2.2. Products
- 9.2.2.3. SWOT Analysis
- 9.2.2.4. Recent Developments
- 9.2.2.5. Financials (Based on Availability)
- 9.2.3 Medtronic PLC
- 9.2.3.1. Overview
- 9.2.3.2. Products
- 9.2.3.3. SWOT Analysis
- 9.2.3.4. Recent Developments
- 9.2.3.5. Financials (Based on Availability)
- 9.2.4 Sonova Holding AG
- 9.2.4.1. Overview
- 9.2.4.2. Products
- 9.2.4.3. SWOT Analysis
- 9.2.4.4. Recent Developments
- 9.2.4.5. Financials (Based on Availability)
- 9.2.5 Getinge AB
- 9.2.5.1. Overview
- 9.2.5.2. Products
- 9.2.5.3. SWOT Analysis
- 9.2.5.4. Recent Developments
- 9.2.5.5. Financials (Based on Availability)
- 9.2.6 Ekso Bionics Holdings Inc
- 9.2.6.1. Overview
- 9.2.6.2. Products
- 9.2.6.3. SWOT Analysis
- 9.2.6.4. Recent Developments
- 9.2.6.5. Financials (Based on Availability)
- 9.2.7 Boston Scientific Corporation
- 9.2.7.1. Overview
- 9.2.7.2. Products
- 9.2.7.3. SWOT Analysis
- 9.2.7.4. Recent Developments
- 9.2.7.5. Financials (Based on Availability)
- 9.2.8 Baxter International Inc
- 9.2.8.1. Overview
- 9.2.8.2. Products
- 9.2.8.3. SWOT Analysis
- 9.2.8.4. Recent Developments
- 9.2.8.5. Financials (Based on Availability)
- 9.2.9 Berlin Heart GmbH
- 9.2.9.1. Overview
- 9.2.9.2. Products
- 9.2.9.3. SWOT Analysis
- 9.2.9.4. Recent Developments
- 9.2.9.5. Financials (Based on Availability)
- 9.2.10 Med-El Elektromedizinische Gerate GmbH
- 9.2.10.1. Overview
- 9.2.10.2. Products
- 9.2.10.3. SWOT Analysis
- 9.2.10.4. Recent Developments
- 9.2.10.5. Financials (Based on Availability)
- 9.2.1 Ossur
List of Figures
- Figure 1: Canada Artificial Organs and Bionics Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Canada Artificial Organs and Bionics Market Share (%) by Company 2024
List of Tables
- Table 1: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Type 2019 & 2032
- Table 4: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Type 2019 & 2032
- Table 5: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 7: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 9: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 11: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 13: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Type 2019 & 2032
- Table 14: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Type 2019 & 2032
- Table 15: Canada Artificial Organs and Bionics Market Revenue Million Forecast, by Country 2019 & 2032
- Table 16: Canada Artificial Organs and Bionics Market Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Canada Artificial Organs and Bionics Market?
The projected CAGR is approximately 6.30%.
2. Which companies are prominent players in the Canada Artificial Organs and Bionics Market?
Key companies in the market include Ossur, Asahi Kasei Medical Co Ltd, Medtronic PLC, Sonova Holding AG, Getinge AB, Ekso Bionics Holdings Inc, Boston Scientific Corporation, Baxter International Inc, Berlin Heart GmbH, Med-El Elektromedizinische Gerate GmbH.
3. What are the main segments of the Canada Artificial Organs and Bionics Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Incidences of Organ Failure; Scarcity of Donor Organs.
6. What are the notable trends driving market growth?
Artificial Kidney Segment is Expected to Witness Significant Growth During the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Procedure; Risk of Compatibility and Malfunctions.
8. Can you provide examples of recent developments in the market?
In September 2022, Axonics completed the first implant of its Axonics F15 sacral neuromodulation (SNM) system for patients in Canada suffering from bladder and bowel dysfunction. The SNS is the only approved implantable bionic solution globally for urinary or bowel incontinence.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Canada Artificial Organs and Bionics Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Canada Artificial Organs and Bionics Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Canada Artificial Organs and Bionics Market?
To stay informed about further developments, trends, and reports in the Canada Artificial Organs and Bionics Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



