Key Insights
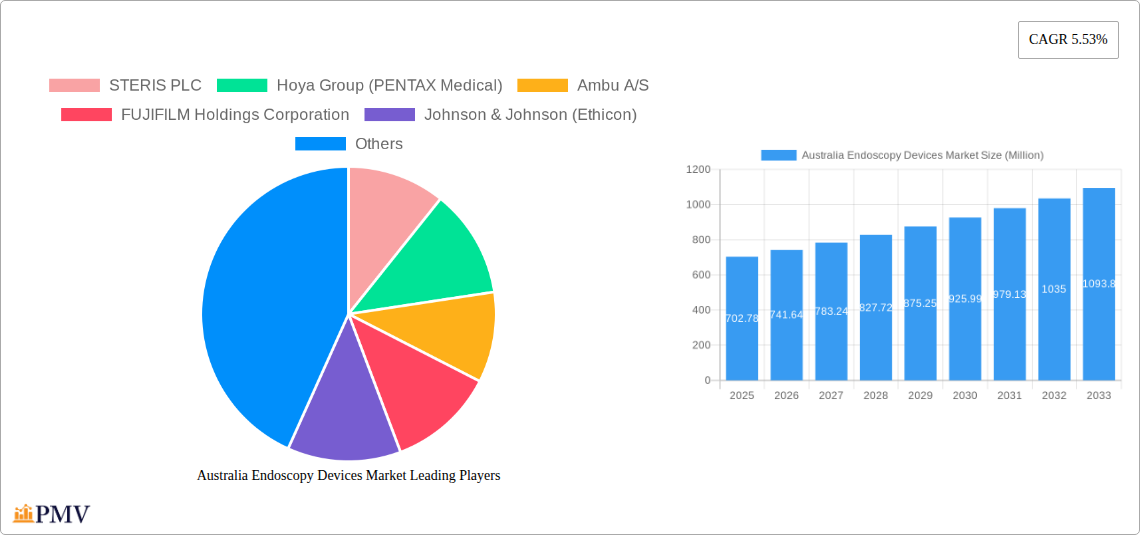
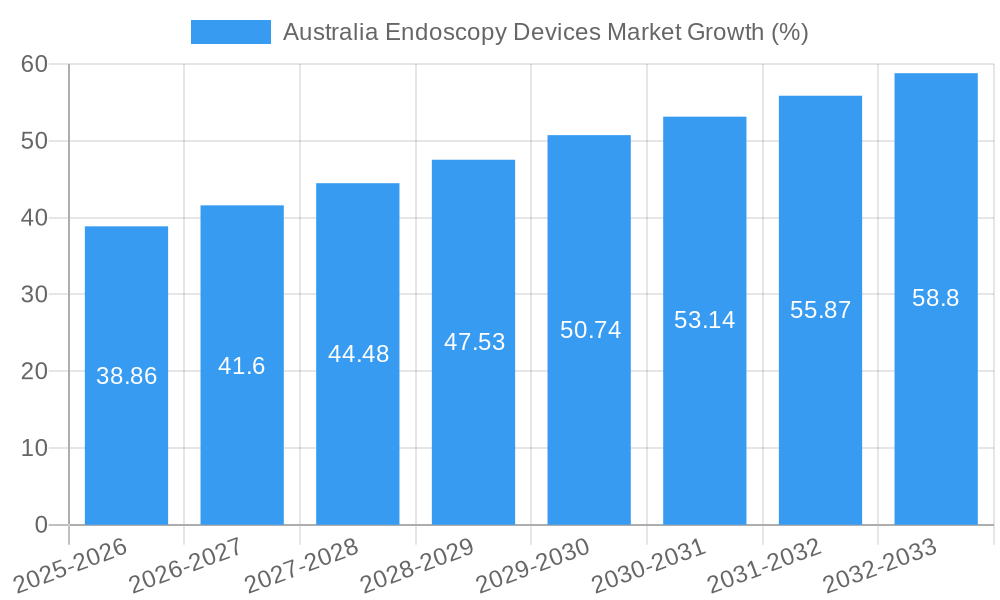
The Australian endoscopy devices market, valued at $702.78 million in 2025, is projected to experience robust growth, driven by a rising prevalence of chronic diseases requiring endoscopic procedures, advancements in minimally invasive surgical techniques, and increasing adoption of technologically advanced endoscopy devices. The market's Compound Annual Growth Rate (CAGR) of 5.53% from 2025 to 2033 indicates a steady expansion, fueled by factors such as an aging population, improved healthcare infrastructure, and growing awareness among both patients and healthcare professionals about the benefits of endoscopic procedures. Significant growth is anticipated in segments like robot-assisted endoscopy, driven by enhanced precision, reduced invasiveness, and improved surgical outcomes. The increasing preference for less-invasive procedures is expected to fuel demand for advanced visualization equipment within the "Other Endoscopic Operative Devices" segment. Gastroenterology remains a dominant application area, but growth is also projected across pulmonology, cardiology, and other surgical specialties. While the market faces potential restraints such as high device costs and the need for skilled professionals, the overall trend points towards considerable expansion, driven by strong technological innovation and an evolving healthcare landscape in Australia.
The competitive landscape is marked by the presence of both established global players and specialized regional companies. Key players like STERIS PLC, Hoya Group (PENTAX Medical), Ambu A/S, and Olympus Corporation are actively engaged in expanding their product portfolios, investing in research and development, and strengthening their distribution networks to cater to the growing demand. Strategic collaborations and partnerships are further accelerating market growth. The market's segmentation by device type (endoscopes, operative devices, visualization equipment) and application (gastroenterology, pulmonology, etc.) allows for granular market analysis and identification of high-growth segments. Future growth will hinge on continued technological advancement, regulatory approvals, and sustained investment in healthcare infrastructure within Australia. Analyzing these trends and competitive dynamics offers crucial insights for stakeholders involved in the Australian endoscopy devices market.
Australia Endoscopy Devices Market: A Comprehensive Report (2019-2033)
This detailed report provides a comprehensive analysis of the Australia endoscopy devices market, offering invaluable insights for industry stakeholders, investors, and researchers. Covering the period from 2019 to 2033, with a base year of 2025, this report offers a historical overview, current market estimations, and future projections. The market is segmented by type of device, application, and device type, providing a granular understanding of its structure and growth trajectory. Key players such as STERIS PLC, Hoya Group (PENTAX Medical), Ambu A/S, FUJIFILM Holdings Corporation, Johnson & Johnson (Ethicon), Karl Storz GmbH & Co KG, Medtronic Plc, B Braun Melsungen AG, Stryker Corporation, and Olympus Corporation are thoroughly analyzed, along with their market share and strategic initiatives. The report also analyzes market drivers, restraints, and future opportunities, offering actionable recommendations for success in this dynamic market.
Australia Endoscopy Devices Market Market Structure & Competitive Dynamics
The Australian endoscopy devices market is characterized by a moderately concentrated structure, with a few major multinational corporations holding significant market share. The market exhibits a dynamic competitive landscape fueled by ongoing technological advancements, strategic partnerships, and mergers and acquisitions (M&A) activities. The regulatory framework, primarily overseen by the Therapeutic Goods Administration (TGA), plays a crucial role in shaping market access and product approvals. Innovation ecosystems are thriving, with significant investments in research and development driving the introduction of advanced endoscopy devices. The market also witnesses competition from substitute technologies, albeit limited, and the increasing demand for minimally invasive procedures fuels market growth. End-user trends such as a preference for advanced visualization and robotic-assisted procedures are significantly influencing market demands.
- Market Concentration: The top five players account for approximately xx% of the market share in 2025.
- M&A Activities: The past five years have witnessed xx M&A deals in the Australian endoscopy devices market, with a total estimated value of xx Million. These transactions have primarily focused on expanding product portfolios and geographic reach.
- Regulatory Landscape: The TGA's stringent regulations influence market entry and product approvals, driving the need for robust clinical trials and regulatory compliance.
- Innovation Ecosystem: Australian universities and research institutions are actively involved in developing new endoscopy technologies, fostering a vibrant innovation ecosystem.
- Product Substitutes: While limited, alternative minimally invasive procedures present a degree of competition.
Australia Endoscopy Devices Market Industry Trends & Insights
The Australian endoscopy devices market is experiencing robust growth, driven by factors such as an aging population, rising prevalence of chronic diseases requiring endoscopic procedures, and increasing adoption of advanced minimally invasive techniques. Technological disruptions, including the integration of artificial intelligence (AI) and robotic assistance, are revolutionizing the field, enhancing procedural accuracy and patient outcomes. Consumer preferences are shifting towards advanced visualization systems, improved ergonomics, and single-use devices to minimize infection risks. The market's competitive intensity is high, with companies focusing on product innovation, strategic partnerships, and acquisitions to gain a competitive edge. The market is expected to exhibit a CAGR of xx% during the forecast period (2025-2033), driven by strong demand across various applications. Market penetration of advanced technologies such as robotic-assisted endoscopes is gradually increasing, although challenges regarding affordability and widespread access remain.
Dominant Markets & Segments in Australia Endoscopy Devices Market
Within the Australian endoscopy devices market, gastroenterology currently holds the largest market share, driven by high prevalence of gastrointestinal disorders and widespread accessibility of endoscopic procedures. Other significant application segments include pulmonology, cardiology, and ENT surgery. The endoscopes segment dominates by type of device due to its wide applicability across various medical procedures. Within the device type segments, other endoscopic operative devices, specifically visualization equipment, are also witnessing significant growth due to increasing demand for high-resolution imaging and advanced visualization capabilities.
By Application:
- Gastroenterology: High prevalence of gastrointestinal disorders and established endoscopic practices drive this segment's dominance.
- Pulmonology: Growing incidence of respiratory diseases is increasing demand for bronchoscopic procedures.
- Other Applications: This segment includes orthopedic surgery, cardiology, ENT surgery, and gynecology, each showing steady growth.
By Type of Device:
- Endoscopes: The core of endoscopic procedures, this segment enjoys consistent growth driven by technological advancements.
- Robot-assisted Endoscope: While still nascent, this segment is showing strong growth potential, driven by improved precision and minimally invasive capabilities.
- Other Endoscopic Operative Devices (Visualization Equipment): Increasing demand for higher quality images boosts this segment.
Key Drivers:
- Growing geriatric population
- Increased prevalence of chronic diseases
- Technological advancements in minimally invasive surgeries
- Government initiatives supporting healthcare infrastructure development
Australia Endoscopy Devices Market Product Innovations
Recent product innovations in the Australian endoscopy devices market are focused on enhancing procedural efficiency, improving visualization, and reducing invasiveness. This includes the development of smaller diameter endoscopes, advanced imaging capabilities, and robotic-assisted systems. Single-use endoscopes are gaining traction due to infection control benefits. These innovations are improving patient outcomes and driving market growth. Competitive advantages are increasingly based on technological superiority, ease of use, and superior image quality.
Report Segmentation & Scope
This report segments the Australia endoscopy devices market based on several key parameters:
By Type of Device: This includes endoscopes, robot-assisted endoscopes (further subdivided into endoscopic operative devices and visualization equipment), and other endoscopic operative devices (including visualization equipment). Each segment shows varying growth rates depending on technological advancements and market adoption.
By Application: This includes gastroenterology, pulmonology, orthopedic surgery, cardiology, ENT surgery, gynecology, and other applications. Each segment’s growth is influenced by the prevalence of related diseases and technological advancements.
Growth projections for each segment are detailed in the full report, along with a comprehensive competitive analysis for each category. Market sizes for 2025 are estimated, while the forecast period extends to 2033, allowing for long-term strategic planning.
Key Drivers of Australia Endoscopy Devices Market Growth
The Australian endoscopy devices market is propelled by several key factors. The aging population contributes to a higher incidence of chronic diseases requiring endoscopic procedures. Technological advancements, such as AI integration and robotic assistance, enhance procedure accuracy and patient outcomes, further driving adoption. Government initiatives aimed at improving healthcare infrastructure and funding also contribute to market expansion. Finally, a growing preference for minimally invasive procedures fuels market growth.
Challenges in the Australia Endoscopy Devices Market Sector
The Australian endoscopy devices market faces several challenges. High costs associated with advanced devices can limit accessibility, especially in rural areas. The stringent regulatory framework can pose barriers to market entry for new players. Supply chain disruptions and potential shortages of raw materials can impact market stability. Finally, intense competition amongst established players necessitates continuous innovation and efficient cost management to maintain profitability.
Leading Players in the Australia Endoscopy Devices Market Market
- STERIS PLC
- Hoya Group (PENTAX Medical)
- Ambu A/S
- FUJIFILM Holdings Corporation
- Johnson & Johnson (Ethicon)
- Karl Storz GmbH & Co KG
- Medtronic Plc
- B Braun Melsungen AG
- Stryker Corporation
- Olympus Corporation
Key Developments in Australia Endoscopy Devices Market Sector
November 2021: Ambu announced early clinical trial results for aScope Duodeno 1.5 showing a 100.0% procedure success rate and planned launch across Australia, Europe, and the United States. This significantly impacts market dynamics by introducing a potentially game-changing product.
April 2021: Endotherapeutics partnered with Cook Medical as a distributor of the Cook Medical Otolaryngology-Head and Neck Surgery (OHNS) clinical specialty portfolio in Australia, including endoscopes. This expands market reach and product availability.
Strategic Australia Endoscopy Devices Market Market Outlook
The future of the Australian endoscopy devices market appears bright, driven by continued technological advancements, an aging population, and increasing demand for minimally invasive procedures. Strategic opportunities exist for companies focusing on innovation, particularly in areas such as AI integration, robotic assistance, and single-use devices. Expansion into underserved rural areas and strategic partnerships with healthcare providers will also be crucial for future success. The market's growth trajectory indicates significant potential for investment and expansion in the coming years.
Australia Endoscopy Devices Market Segmentation
-
1. Type of Device
- 1.1. Endoscopes
- 1.2. Endoscopic Operative Device
- 1.3. Visualization Equipment
-
2. Application
- 2.1. Gastroenterology
- 2.2. Pulmonology
- 2.3. Orthopedic Surgery
- 2.4. Cardiology
- 2.5. ENT Surgery
- 2.6. Gynecology
- 2.7. Other Applications
Australia Endoscopy Devices Market Segmentation By Geography
- 1. Australia
Australia Endoscopy Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.53% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Preference for Minimally Invasive Surgeries; Rise in the Prevalence of Diseases that Require Endoscopy Procedures
- 3.3. Market Restrains
- 3.3.1. High Cost of Sophisticated Endoscopy Devices
- 3.4. Market Trends
- 3.4.1. Cardiology Segment is Expected to Witness a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Australia Endoscopy Devices Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 5.1.1. Endoscopes
- 5.1.2. Endoscopic Operative Device
- 5.1.3. Visualization Equipment
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Gastroenterology
- 5.2.2. Pulmonology
- 5.2.3. Orthopedic Surgery
- 5.2.4. Cardiology
- 5.2.5. ENT Surgery
- 5.2.6. Gynecology
- 5.2.7. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Australia
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2024
- 6.2. Company Profiles
- 6.2.1 STERIS PLC
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Hoya Group (PENTAX Medical)
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Ambu A/S
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 FUJIFILM Holdings Corporation
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Johnson & Johnson (Ethicon)
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Karl Storz GmbH & Co KG
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Medtronic Plc
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 B Braun Melsungen AG
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.9 Stryker Corporation
- 6.2.9.1. Overview
- 6.2.9.2. Products
- 6.2.9.3. SWOT Analysis
- 6.2.9.4. Recent Developments
- 6.2.9.5. Financials (Based on Availability)
- 6.2.10 Olympus Corporation
- 6.2.10.1. Overview
- 6.2.10.2. Products
- 6.2.10.3. SWOT Analysis
- 6.2.10.4. Recent Developments
- 6.2.10.5. Financials (Based on Availability)
- 6.2.1 STERIS PLC
List of Figures
- Figure 1: Australia Endoscopy Devices Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Australia Endoscopy Devices Market Share (%) by Company 2024
List of Tables
- Table 1: Australia Endoscopy Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Australia Endoscopy Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Australia Endoscopy Devices Market Revenue Million Forecast, by Type of Device 2019 & 2032
- Table 4: Australia Endoscopy Devices Market Volume K Unit Forecast, by Type of Device 2019 & 2032
- Table 5: Australia Endoscopy Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 6: Australia Endoscopy Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 7: Australia Endoscopy Devices Market Revenue Million Forecast, by Region 2019 & 2032
- Table 8: Australia Endoscopy Devices Market Volume K Unit Forecast, by Region 2019 & 2032
- Table 9: Australia Endoscopy Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Australia Endoscopy Devices Market Volume K Unit Forecast, by Country 2019 & 2032
- Table 11: Australia Endoscopy Devices Market Revenue Million Forecast, by Type of Device 2019 & 2032
- Table 12: Australia Endoscopy Devices Market Volume K Unit Forecast, by Type of Device 2019 & 2032
- Table 13: Australia Endoscopy Devices Market Revenue Million Forecast, by Application 2019 & 2032
- Table 14: Australia Endoscopy Devices Market Volume K Unit Forecast, by Application 2019 & 2032
- Table 15: Australia Endoscopy Devices Market Revenue Million Forecast, by Country 2019 & 2032
- Table 16: Australia Endoscopy Devices Market Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Australia Endoscopy Devices Market?
The projected CAGR is approximately 5.53%.
2. Which companies are prominent players in the Australia Endoscopy Devices Market?
Key companies in the market include STERIS PLC, Hoya Group (PENTAX Medical), Ambu A/S, FUJIFILM Holdings Corporation, Johnson & Johnson (Ethicon), Karl Storz GmbH & Co KG, Medtronic Plc, B Braun Melsungen AG, Stryker Corporation, Olympus Corporation.
3. What are the main segments of the Australia Endoscopy Devices Market?
The market segments include Type of Device, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD 702.78 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Preference for Minimally Invasive Surgeries; Rise in the Prevalence of Diseases that Require Endoscopy Procedures.
6. What are the notable trends driving market growth?
Cardiology Segment is Expected to Witness a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Sophisticated Endoscopy Devices.
8. Can you provide examples of recent developments in the market?
In November 2021, Ambu announced early clinical trial results for aScope Duodeno 1.5 showing a 100.0% procedure success rate and was planning to launch across Australia, Europe, and the United States.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Australia Endoscopy Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Australia Endoscopy Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Australia Endoscopy Devices Market?
To stay informed about further developments, trends, and reports in the Australia Endoscopy Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



