Key Insights
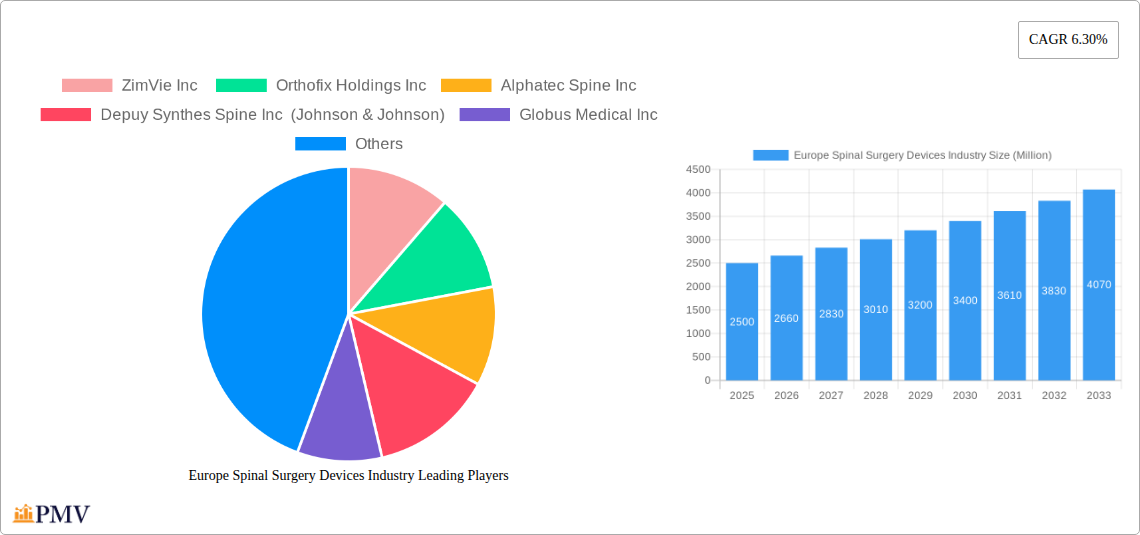
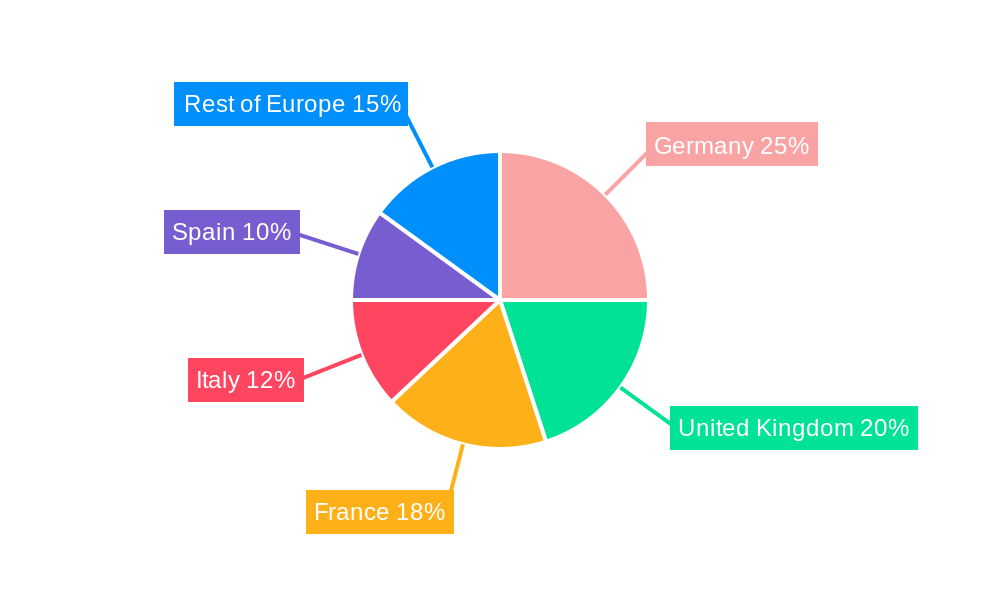
The European spinal surgery devices market, valued at approximately €X billion in 2025 (assuming a reasonable market size based on global trends and the provided CAGR), is projected to experience robust growth, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2033. This expansion is fueled by several key drivers. The aging population across Europe is leading to a higher incidence of degenerative spinal conditions requiring surgical intervention. Technological advancements in minimally invasive spinal surgery techniques, such as robotic-assisted surgery and advanced imaging technologies, are improving surgical precision and patient outcomes, thus driving market demand. Furthermore, increasing awareness of spinal health and the availability of advanced implants like those made from PEEK and titanium are contributing to market growth. The market is segmented by device type (spinal decompression, fusion, arthroplasty, non-fusion), material (titanium, PEEK, ceramic), and end-user (hospitals, clinics, orthopedic centers). Germany, the United Kingdom, France, Italy, and Spain represent the largest national markets within Europe.
However, the market faces certain restraints. High costs associated with spinal surgery devices and procedures can limit accessibility, particularly in countries with strained healthcare systems. Stringent regulatory approvals for new devices can also impede market entry and growth. Nevertheless, the overall market outlook remains positive, driven by the aforementioned factors and the continuous development of innovative spinal implants and surgical techniques catering to an aging population with increasingly prevalent spinal conditions. The competitive landscape includes major players such as ZimVie Inc, Orthofix Holdings Inc, Alphatec Spine Inc, and others, continuously striving for innovation and market share. The forecast period suggests a significant expansion of the market, with considerable opportunities for both established players and emerging companies focusing on innovative technologies and improved patient outcomes.
Europe Spinal Surgery Devices Industry: A Comprehensive Market Report (2019-2033)
This detailed report provides a comprehensive analysis of the Europe spinal surgery devices market, offering invaluable insights for stakeholders across the industry value chain. Spanning the period from 2019 to 2033, with a focus on 2025, this report meticulously examines market structure, competitive dynamics, technological advancements, and future growth prospects. The report is crucial for understanding the complexities of this rapidly evolving sector and making informed business decisions.
Europe Spinal Surgery Devices Industry Market Structure & Competitive Dynamics
The European spinal surgery devices market is characterized by a moderately consolidated structure with a mix of multinational giants and specialized players. Key players like ZimVie Inc, Orthofix Holdings Inc, Alphatec Spine Inc, Depuy Synthes Spine Inc (Johnson & Johnson), Globus Medical Inc, Medtronic PLC, SeaSpine Inc, SpineGuard SA, Stryker Corporation, and NuVasive Inc hold significant market share, driving innovation and competition. Market concentration is estimated at xx%, with the top 5 players accounting for approximately xx% of the total market revenue in 2025. The industry is shaped by stringent regulatory frameworks like the MDR (Medical Device Regulation) and active innovation ecosystems, fostering the development of novel spinal implants and surgical techniques. The prevalence of substitute therapies (e.g., non-surgical treatments) also influences market dynamics. End-user trends are shifting toward minimally invasive procedures and personalized medicine, necessitating the development of advanced devices. Mergers and acquisitions (M&A) are a prominent feature, with recent activity focused on expanding product portfolios and geographical reach. For example, the xx Million acquisition of Spine Innovations by Spineway in July 2022 showcases this trend. Further M&A deals are expected, with projected deal values exceeding xx Million in the forecast period (2025-2033).
Europe Spinal Surgery Devices Industry Industry Trends & Insights
The European spinal surgery devices market is experiencing robust growth, driven by factors such as an aging population, rising prevalence of spinal disorders (degenerative disc disease, scoliosis, trauma), increasing awareness, and advancements in surgical techniques and implant technologies. The market is expected to exhibit a CAGR of xx% during the forecast period (2025-2033). Technological disruptions, such as the adoption of 3D printing for customized implants and the integration of robotics in spinal surgery, are revolutionizing the landscape. Consumer preferences are increasingly focused on minimally invasive procedures, shorter recovery times, and improved long-term outcomes. This trend fuels demand for innovative devices that minimize trauma and enhance patient experience. The competitive landscape remains dynamic, with ongoing innovation, strategic partnerships, and product launches shaping the market trajectory. Market penetration of advanced technologies like spinal navigation systems is steadily increasing, projected to reach xx% by 2033. The growing emphasis on value-based healthcare is also impacting market dynamics, incentivizing the development of cost-effective and clinically effective solutions.
Dominant Markets & Segments in Europe Spinal Surgery Devices Industry
The German market currently holds a leading position within Europe, attributed to robust healthcare infrastructure, high prevalence of spinal disorders, and significant investments in healthcare technology. Other key markets include the UK, France, and Italy.
Device Type: Spinal fusion procedures dominate the market, accounting for the largest revenue share. However, the segment for non-fusion procedures and spinal decompression is experiencing faster growth due to rising demand for less invasive options. Arthroplasty is also a significant segment with increasing acceptance.
Material: Titanium remains the dominant material due to its biocompatibility and strength. However, the use of PEEK (polyetheretherketone) is expanding rapidly, driven by its superior mechanical properties and radiolucency. Ceramic materials are gaining traction in specialized applications.
End-User: Hospitals and specialized orthopedic centers are the primary end-users, representing a significant proportion of market revenue. The increasing prevalence of ambulatory surgery centers is expected to drive growth in this segment.
Key drivers contributing to the dominance of specific segments and markets include:
- Robust healthcare infrastructure: Countries with well-developed healthcare systems tend to see higher adoption rates.
- Favorable reimbursement policies: Government policies that support the use of advanced spinal devices influence market growth.
- High prevalence of spinal disorders: A greater prevalence of spinal conditions in the population translates into higher demand.
Europe Spinal Surgery Devices Industry Product Innovations
Recent advancements focus on minimally invasive techniques, improved biomaterials, and personalized implants. 3D-printed implants tailored to individual patient anatomy are gaining popularity, alongside advanced navigation systems that enhance surgical precision. The integration of smart sensors and data analytics is also transforming the field, improving patient outcomes and facilitating remote monitoring. These innovations are addressing market needs for less invasive procedures, shorter recovery times, and improved patient experiences. The development of bio-resorbable implants is an area of ongoing innovation which is expected to contribute to xx Million in additional revenue by 2033.
Report Segmentation & Scope
This report segments the European spinal surgery devices market based on device type (Spinal Decompression, Spinal Fusion, Arthroplasty, Non-fusion Procedures), material (Titanium, PEEK, Ceramic), and end-user (Hospitals, Clinics, Orthopedic Centers). Each segment is analyzed in detail, providing market size estimations, growth projections, and competitive dynamics. The forecast period is 2025-2033, with 2025 serving as the base year. The report also provides historical data from 2019-2024. Specific growth rates vary across the segments, with xx% being the average growth for the total market. The competitive landscape within each segment is analyzed individually. For instance, the spinal fusion segment is dominated by a few major players while the non-fusion procedures market presents an opportunity for newer companies to enter.
Key Drivers of Europe Spinal Surgery Devices Industry Growth
The European spinal surgery devices market's growth is propelled by several factors: an aging population leading to increased prevalence of spinal disorders, technological advancements (minimally invasive surgery, smart implants), increasing healthcare expenditure, and favorable regulatory environments supporting innovation. Furthermore, rising awareness of spinal health and improved access to healthcare contribute to market expansion. The increasing adoption of value-based care models is creating opportunities for manufacturers to demonstrate clinical and cost-effectiveness.
Challenges in the Europe Spinal Surgery Devices Industry Sector
The industry faces challenges including stringent regulatory approvals for new devices, complexities in healthcare reimbursement systems, intense competition among established players and new entrants, and supply chain vulnerabilities impacting material availability and device production. These factors can lead to delays in product launches, increased costs, and reduced profitability. The impact of these challenges is projected to be a xx% reduction in overall growth in the short-term.
Leading Players in the Europe Spinal Surgery Devices Industry Market
- ZimVie Inc
- Orthofix Holdings Inc
- Alphatec Spine Inc
- Depuy Synthes Spine Inc (Johnson & Johnson)
- Globus Medical Inc
- Medtronic PLC
- SeaSpine Inc
- SpineGuard SA
- Stryker Corporation
- NuVasive Inc
Key Developments in Europe Spinal Surgery Devices Industry Sector
- July 2022: Spineway acquired Spine Innovations, expanding its product portfolio in cervical and lumbar disc prostheses, increasing market share within the French market.
- June 2022: NGMedical GmbH launched the ART Fixation System, introducing a new product into the European market, potentially impacting the competition dynamics.
Strategic Europe Spinal Surgery Devices Industry Market Outlook
The future of the European spinal surgery devices market looks promising, driven by ongoing technological innovation, expanding applications of minimally invasive techniques, and the growing adoption of personalized medicine. Strategic opportunities exist for companies that can effectively leverage digital technologies, create innovative products, and adapt to evolving regulatory and reimbursement landscapes. The market presents significant potential for growth, particularly in segments focused on less invasive techniques and advanced materials, promising continued expansion through the forecast period.
Europe Spinal Surgery Devices Industry Segmentation
-
1. Device Type
-
1.1. Spinal Decompression
- 1.1.1. Corpectomy
- 1.1.2. Discectomy
- 1.1.3. Facetectomy
- 1.1.4. Foraminotomy
- 1.1.5. Laminotomy
-
1.2. Spinal Fusion
- 1.2.1. Cervical Fusion
- 1.2.2. Interbody Fusion
- 1.2.3. Thoraco Lumbar Fusion
- 1.2.4. Other Spinal Fusions
- 1.3. Fracture Repair
- 1.4. Arthroplasty
- 1.5. Non-fusion Procedures
-
1.1. Spinal Decompression
Europe Spinal Surgery Devices Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Spinal Surgery Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.30% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity
- 3.2.2 Aging Population
- 3.2.3 and Associated Spine Disorders; Continuous Advancements in Spine Surgery Technologies
- 3.3. Market Restrains
- 3.3.1. High Cost and Time Involved in Treatment Procedures; Reimbursement Issues
- 3.4. Market Trends
- 3.4.1. Lumbar Fusion is Expected to Witness a Significant Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Spinal Decompression
- 5.1.1.1. Corpectomy
- 5.1.1.2. Discectomy
- 5.1.1.3. Facetectomy
- 5.1.1.4. Foraminotomy
- 5.1.1.5. Laminotomy
- 5.1.2. Spinal Fusion
- 5.1.2.1. Cervical Fusion
- 5.1.2.2. Interbody Fusion
- 5.1.2.3. Thoraco Lumbar Fusion
- 5.1.2.4. Other Spinal Fusions
- 5.1.3. Fracture Repair
- 5.1.4. Arthroplasty
- 5.1.5. Non-fusion Procedures
- 5.1.1. Spinal Decompression
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Germany
- 5.2.2. United Kingdom
- 5.2.3. France
- 5.2.4. Italy
- 5.2.5. Spain
- 5.2.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Germany Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Spinal Decompression
- 6.1.1.1. Corpectomy
- 6.1.1.2. Discectomy
- 6.1.1.3. Facetectomy
- 6.1.1.4. Foraminotomy
- 6.1.1.5. Laminotomy
- 6.1.2. Spinal Fusion
- 6.1.2.1. Cervical Fusion
- 6.1.2.2. Interbody Fusion
- 6.1.2.3. Thoraco Lumbar Fusion
- 6.1.2.4. Other Spinal Fusions
- 6.1.3. Fracture Repair
- 6.1.4. Arthroplasty
- 6.1.5. Non-fusion Procedures
- 6.1.1. Spinal Decompression
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. United Kingdom Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Spinal Decompression
- 7.1.1.1. Corpectomy
- 7.1.1.2. Discectomy
- 7.1.1.3. Facetectomy
- 7.1.1.4. Foraminotomy
- 7.1.1.5. Laminotomy
- 7.1.2. Spinal Fusion
- 7.1.2.1. Cervical Fusion
- 7.1.2.2. Interbody Fusion
- 7.1.2.3. Thoraco Lumbar Fusion
- 7.1.2.4. Other Spinal Fusions
- 7.1.3. Fracture Repair
- 7.1.4. Arthroplasty
- 7.1.5. Non-fusion Procedures
- 7.1.1. Spinal Decompression
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. France Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Spinal Decompression
- 8.1.1.1. Corpectomy
- 8.1.1.2. Discectomy
- 8.1.1.3. Facetectomy
- 8.1.1.4. Foraminotomy
- 8.1.1.5. Laminotomy
- 8.1.2. Spinal Fusion
- 8.1.2.1. Cervical Fusion
- 8.1.2.2. Interbody Fusion
- 8.1.2.3. Thoraco Lumbar Fusion
- 8.1.2.4. Other Spinal Fusions
- 8.1.3. Fracture Repair
- 8.1.4. Arthroplasty
- 8.1.5. Non-fusion Procedures
- 8.1.1. Spinal Decompression
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Italy Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Spinal Decompression
- 9.1.1.1. Corpectomy
- 9.1.1.2. Discectomy
- 9.1.1.3. Facetectomy
- 9.1.1.4. Foraminotomy
- 9.1.1.5. Laminotomy
- 9.1.2. Spinal Fusion
- 9.1.2.1. Cervical Fusion
- 9.1.2.2. Interbody Fusion
- 9.1.2.3. Thoraco Lumbar Fusion
- 9.1.2.4. Other Spinal Fusions
- 9.1.3. Fracture Repair
- 9.1.4. Arthroplasty
- 9.1.5. Non-fusion Procedures
- 9.1.1. Spinal Decompression
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. Spain Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Spinal Decompression
- 10.1.1.1. Corpectomy
- 10.1.1.2. Discectomy
- 10.1.1.3. Facetectomy
- 10.1.1.4. Foraminotomy
- 10.1.1.5. Laminotomy
- 10.1.2. Spinal Fusion
- 10.1.2.1. Cervical Fusion
- 10.1.2.2. Interbody Fusion
- 10.1.2.3. Thoraco Lumbar Fusion
- 10.1.2.4. Other Spinal Fusions
- 10.1.3. Fracture Repair
- 10.1.4. Arthroplasty
- 10.1.5. Non-fusion Procedures
- 10.1.1. Spinal Decompression
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. Rest of Europe Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 11.1.1. Spinal Decompression
- 11.1.1.1. Corpectomy
- 11.1.1.2. Discectomy
- 11.1.1.3. Facetectomy
- 11.1.1.4. Foraminotomy
- 11.1.1.5. Laminotomy
- 11.1.2. Spinal Fusion
- 11.1.2.1. Cervical Fusion
- 11.1.2.2. Interbody Fusion
- 11.1.2.3. Thoraco Lumbar Fusion
- 11.1.2.4. Other Spinal Fusions
- 11.1.3. Fracture Repair
- 11.1.4. Arthroplasty
- 11.1.5. Non-fusion Procedures
- 11.1.1. Spinal Decompression
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 12. Germany Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 13. United Kingdom Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 14. France Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 15. Italy Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 16. Spain Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 17. Rest of Europe Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 18. Competitive Analysis
- 18.1. Market Share Analysis 2024
- 18.2. Company Profiles
- 18.2.1 ZimVie Inc
- 18.2.1.1. Overview
- 18.2.1.2. Products
- 18.2.1.3. SWOT Analysis
- 18.2.1.4. Recent Developments
- 18.2.1.5. Financials (Based on Availability)
- 18.2.2 Orthofix Holdings Inc
- 18.2.2.1. Overview
- 18.2.2.2. Products
- 18.2.2.3. SWOT Analysis
- 18.2.2.4. Recent Developments
- 18.2.2.5. Financials (Based on Availability)
- 18.2.3 Alphatec Spine Inc
- 18.2.3.1. Overview
- 18.2.3.2. Products
- 18.2.3.3. SWOT Analysis
- 18.2.3.4. Recent Developments
- 18.2.3.5. Financials (Based on Availability)
- 18.2.4 Depuy Synthes Spine Inc (Johnson & Johnson)
- 18.2.4.1. Overview
- 18.2.4.2. Products
- 18.2.4.3. SWOT Analysis
- 18.2.4.4. Recent Developments
- 18.2.4.5. Financials (Based on Availability)
- 18.2.5 Globus Medical Inc
- 18.2.5.1. Overview
- 18.2.5.2. Products
- 18.2.5.3. SWOT Analysis
- 18.2.5.4. Recent Developments
- 18.2.5.5. Financials (Based on Availability)
- 18.2.6 Medtronic PLC
- 18.2.6.1. Overview
- 18.2.6.2. Products
- 18.2.6.3. SWOT Analysis
- 18.2.6.4. Recent Developments
- 18.2.6.5. Financials (Based on Availability)
- 18.2.7 SeaSpine Inc
- 18.2.7.1. Overview
- 18.2.7.2. Products
- 18.2.7.3. SWOT Analysis
- 18.2.7.4. Recent Developments
- 18.2.7.5. Financials (Based on Availability)
- 18.2.8 SpineGuard SA
- 18.2.8.1. Overview
- 18.2.8.2. Products
- 18.2.8.3. SWOT Analysis
- 18.2.8.4. Recent Developments
- 18.2.8.5. Financials (Based on Availability)
- 18.2.9 Stryker Corporation
- 18.2.9.1. Overview
- 18.2.9.2. Products
- 18.2.9.3. SWOT Analysis
- 18.2.9.4. Recent Developments
- 18.2.9.5. Financials (Based on Availability)
- 18.2.10 NuVasive Inc
- 18.2.10.1. Overview
- 18.2.10.2. Products
- 18.2.10.3. SWOT Analysis
- 18.2.10.4. Recent Developments
- 18.2.10.5. Financials (Based on Availability)
- 18.2.1 ZimVie Inc
List of Figures
- Figure 1: Europe Spinal Surgery Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Spinal Surgery Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 3: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 4: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 5: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 7: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 9: Germany Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Germany Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 11: United Kingdom Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: United Kingdom Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 13: France Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: France Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 15: Italy Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Italy Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 17: Spain Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Spain Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 19: Rest of Europe Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Rest of Europe Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 21: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 22: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 23: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 24: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 25: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 26: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 27: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 29: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 30: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 31: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 32: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 33: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 34: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 35: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 36: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 37: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 38: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 39: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 40: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 41: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 42: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 43: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 44: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Spinal Surgery Devices Industry?
The projected CAGR is approximately 6.30%.
2. Which companies are prominent players in the Europe Spinal Surgery Devices Industry?
Key companies in the market include ZimVie Inc , Orthofix Holdings Inc, Alphatec Spine Inc, Depuy Synthes Spine Inc (Johnson & Johnson), Globus Medical Inc, Medtronic PLC, SeaSpine Inc, SpineGuard SA, Stryker Corporation, NuVasive Inc.
3. What are the main segments of the Europe Spinal Surgery Devices Industry?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity. Aging Population. and Associated Spine Disorders; Continuous Advancements in Spine Surgery Technologies.
6. What are the notable trends driving market growth?
Lumbar Fusion is Expected to Witness a Significant Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost and Time Involved in Treatment Procedures; Reimbursement Issues.
8. Can you provide examples of recent developments in the market?
July 2022: Spineway, a specialist in innovative implants for the treatment of severe spine pathologies, completed the acquisition of 100% of the capital of the French company Spine Innovations, which specializes in cervical and lumbar disc prostheses.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Spinal Surgery Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Spinal Surgery Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Spinal Surgery Devices Industry?
To stay informed about further developments, trends, and reports in the Europe Spinal Surgery Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database


Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence



